Extract from Summary of the ME Association meeting of the Board of Trustees which was held on 15 and 16 November and at which the issue of the controversial Bath/Bristol Lightning Process pilot study in children was discussed.
Shortlink: http://wp.me/p5foE-3eX
http://www.meassociation.org.uk/?p=3059
Summary of the MEA Board of Trustees meeting, November 2010
by tonybritton on November 20, 2010This is a summary of key points to emerge from two routine meetings of The ME Association Board of Trustees.
These meetings took place at our Head Office in Buckingham on Monday afternoon, November 15th and on Tuesday morning, November 16th.
Informal discussion on some of the topics also occurred on the Monday morning.
This is a summary of the two Board meetings – not the official minutes.
The order of subjects below is not necessarily in the order that they were discussed.
Where appropriate, there is background information relating to the issue being discussed.
[…]
Lightning Process:
Trustees held a further discussion on a controversial new research study that has been announced into the use of the Lightning Process. Costing £164,000, the feasibility study will investigate how children and adolescents could be involved in a randomised controlled trial that will assess the Lightning Process and compare it to specialist medical care. Not surprisingly, a number of concerns and objections have been raised about the possible use of children and adolescents in this type of study and we are discussing these concerns with our colleagues in other ME/CFS charities. As a result of these discussion The MEA and the Young ME Sufferers Trust (Tymes Trust) issued a joint statement of concern: http://www.meassociation.org.uk/?page_id=1341
This statement was sent to the Department of Health with a request that it should be forwarded to the ethics committee that is dealing with the application but the DoH refused to do so. Following a Freedom of Information request we obtained the identify the ethics committee that was dealing with the application and our statement was then forwarded to the Chairman. Unfortunately, due to initial secrecy surrounding the identity of the ethics committee, the information did not reach them till after the application had been approved.
We have also passed our concerns to the National Research Ethics Service, who are considering whether the local ethics committee should review their decision. More information can be found on the MEA website: http://www.meassociation.org.uk/?p=2720
A BBC radio discussion from Thursday 11 November about the Lightning Process – which included contributions from Professor Leslie Findley, Dr Charles Shepherd and Phil Parker and was chaired by Anne Diamond – can be heard on YouTube: http://www.meassociation.org.uk/?p=2921. A transcript is also available on the MEA website.
[Extract ends]
A few points:
The Bath/Bristol press release announcing the pilot study was published on 2 March 2010.
The ME Association and The Young ME Sufferers Trust did not issue a joint statement and press release condemning the pilot study until five months later, on 4 August.
Under FOI (in a response dated 27 August) I established that the REC responsible for reviewing the application was the South West 2 Research Ethics Committee. This information was provided by Jonathan Cramp, FOI Manager, NHS South West.
This information had been requested by me of the University of Bristol on 15 May but was denied in a response of 17 June.
It was denied a second time following a request for an Internal Review of the University’s decision to withhold almost all information that had been requested by me under the FOIA. The Internal Review was handled by Sue Paterson, Director of Legal Services and Deputy Secretary, Office of the University Secretary, from whom a response was received on 17 August.
Within the response of Jonathan Cramp, FOI Manager, NHS South West, was the information that the application for ethics approval had been received on 14 June 2010 and that South West 2 RE committee had met to consider the application on 08 July 2010.
As soon as I had received confirmation of the name of the REC which had (already) reviewed the application, this was passed to the ME Association, who had also been passed all previous communications I had been having with various FOI offices, with various parliamentarians, with the Department of Health and with the South West Regional Manager, National Research Ethics Service.
As recorded in the MEA’s meeting summary, above, it was the case that by the time it had been established which RE committee had reviewed the application for ethics approval, the committee had already met six or seven weeks previously (although a favourable opinion was not handed down until mid September since the CI had been asked to revise some content of the patient literature and also address other areas of concern which delayed a decision).
When the MEA and TYMES Trust did issue a statement on 4 August, condemning the pilot and calling for the study to be abandoned, this was widely welcomed, as has been the ME Association’s initiative in contacting Trading Standards offices, as is their continued interest in this issue.
It remains unclear, though, and of concern to me, why these two patient groups took five months to issue position statements and a joint press release.
RNHRD NHS FT Bath/University of Bristol Lightning Process pilot study in children 12 to 18 (SMILE study): Minutes of meeting of External Advisory Group held on 02.11.10: http://wp.me/p5foE-3er
Transcript BBC Radio Berkshire Anne Diamond Show, broadcast 11 November 2010: http://wp.me/p5foE-3dG
Related information:
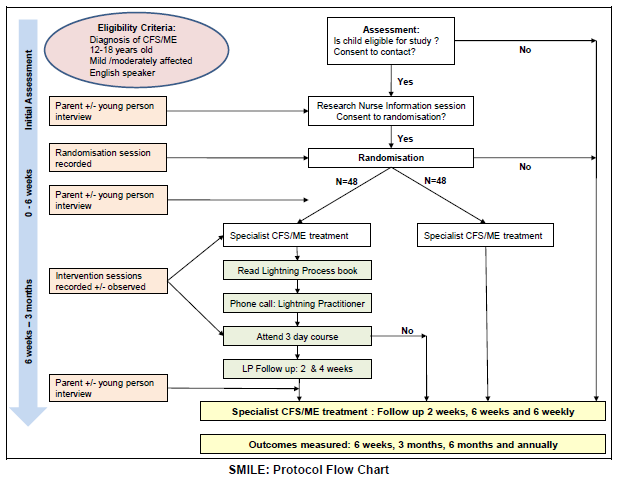
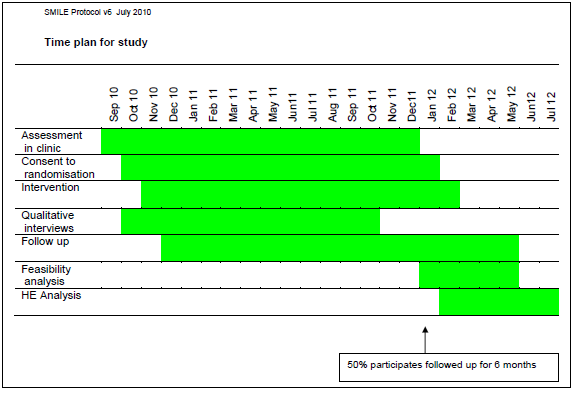
1] SMILE – Specialist Medical Intervention and Lightning Evaluation documents (Lightning Process pilot study – children [now aged 12 to 18] with CFS and ME): http://wp.me/p5foE-37x
2] Background to this issue: http://wp.me/p5foE-2Vt
3] All posts on Lightning Process pilot study in children issue: https://meagenda.wordpress.com/category/lightning-process-smile-study/